

Note: FPD-first patient dosed
CLINICAL-STAGE PIPELINE
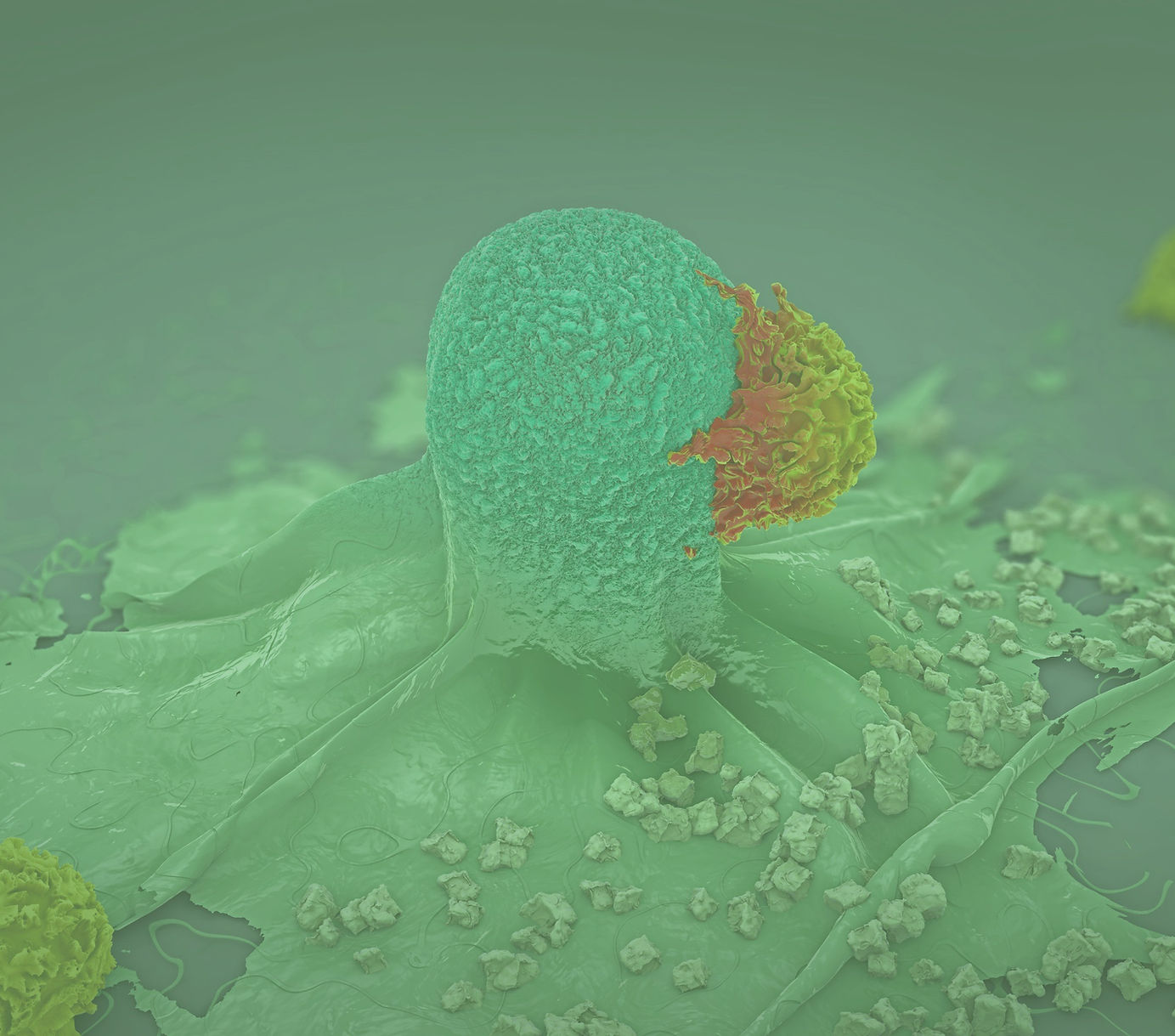
Published scientific evidence links GM-CSF expression to serious and potentially life-threatening outcomes in respiratory conditions such as COVID-19 pneumonia. Evidence also indicates a potential role of GM-CSF expression in serious and potentially life-threatening side-effects associated with CAR-T therapy, and reduced efficacy in CAR-T therapies approved by the US Food and Drug Administration (“FDA”). As a result, while we believe our leadership position in GM-CSF pathway science and cytokine storm presents us with a diverse set of development opportunities, we, along with our partners, currently are focused on developing lenzilumab for four primary indications:
-
As a therapy treating severe and critical hospitalized patients with confirmed COVID-19 pneumonia, which is the majority of hospitalized patients who are hypoxic and not yet on invasive mechanical ventilation (“IMV”);
-
As a prophylactic therapy ahead of CAR-T administration in CD19 targeted CAR-T therapies;
-
As an early treatment or potential prophylaxis for acute GvHD following HSCT in high and intermediate risk patients;
-
As a treatment for chronic myelomonocytic leukemia in patients with RAS pathway mutations
In addition to these programs with lenzilumab, we are also exploring the effectiveness of our GM-CSF neutralization technologies (either through the use of lenzilumab as a neutralizing antibody, or through GM-CSF gene knockout) in combination with other CAR-T, T-cell engaging, and immunotherapy treatments to break the efficacy/toxicity linkage. Our efforts to develop lenzilumab have included four completed studies, the largest of which is our Phase 3 LIVE-AIR study in COVID-19 patients. No treatment emergent serious adverse events (SAEs) or Suspected Unexpected Serious Adverse Reactions (SUSARSs) have been reported across any of the four completed clinical development studies of lenzilumab.
Our clinical-stage pipeline also comprises a further Phase I study with ifabotuzumab (“ifab”) in glioblastoma multiforme (“GBM”). The GBM study is nearly fully enrolled. We believe ifab may have potential in other solid cancers. We also have a focus on creating safer and more effective CAR-T therapies in hematologic malignancies and solid tumors via three key modalities:
-
Combining FDA-approved and development stage CAR-T therapies with lenzilumab;
-
Creating next-generation gene-edited CAR-T therapies using GM-CSF gene knockout technologies; and
-
Exploring the effectiveness of our GM-CSF neutralization technologies (either through the use of lenzilumab as a neutralizing antibody or through GM-CSF gene knockout) in combination with other CAR-T, T-cell engaging, and immunotherapy treatments, including allogeneic HSCT.
These product candidates are in the early stage of development and will require substantial time, resources, research and development, and regulatory approval prior to commercialization. Furthermore, none of these product candidates have been approved for marketing and it may be years, if this occurs at all.
We believe that we have built an intellectual property position in the area of GM-CSF neutralization through multiple approaches and mechanisms, as they pertain to COVID-19, CAR-T, GvHD and multiple other oncology/transplantation, inflammation, fibrosis and autoimmune conditions which may be driven by GM-CSF.