Humanigen has discovered, developed, and plans to commercialize LENZ® (lenzilumab), a variant-agnostic immunomodulatory antibody, which addresses a significant unmet need in COVID-19.
LENZ may offer a ‘future-proof’ treatment against current and inevitable future variants. We believe that COVID-19 will become a serious endemic disease and will continue to impact society, healthcare systems and patients.
LENZ is an effective therapeutic which has demonstrated significant benefit over and above existing standard of care and, importantly, with a safety profile comparable to placebo1.
LENZ could potentially save countless lives, generate billions of dollars in recurring annual revenue, while also offering healthcare systems a cost-effective medicine2 and in our view could be an essential countermeasure for government stockpiling.
LENZ is poised to enter a registrational study in CAR-T and a potential registrational study in acute Graft versus Host Disease, areas of significant unmet need for patients, healthcare professionals and payers, while generating substantial additional value.
Humanigen, Inc. is a clinical stage biopharmaceutical company, developing a portfolio of proprietary immuno-oncology and immunolomodulatory antibodies. We are focusing our efforts on the development of our lead product candidate, LENZ, our Humaneered® (“Humaneered”) anti-human granulocyte-macrophage colony-stimulating factor (“GM-CSF”) immunomodulatory antibody. LENZ has been demonstrated to neutralize human GM-CSF, a cytokine of critical importance in the hyperinflammatory cascade, sometimes referred to as cytokine release syndrome (“CRS”) or “cytokine storm”. GM-CSF neutralization with LENZ has been shown to reduce downstream inflammatory cytokines, prevent hyperinflammation and reduce progression to mechanical ventilation and death in COVID-19 patients.
LENZ has successfully completed a 520-patient phase 3 study (“LIVE-AIR”). There was also a time saved to recovery (2 days), in ICU (3 days) and on IMV (3 days). The observed benefit of LENZ was over and above any benefit provided by steroids and/or remdesivir. In LIVE-AIR, LENZ was safe and well-tolerated, there were no serious adverse events attributed to LENZ and it was noted to have a safety profile comparable to placebo, a profile consistent with other clinical studies conducted with LENZ. The positive LIVE-AIR results have been published with and accompanying expert opinion in Lancet Respiratory Medicine.
The NIH’s ACTIV-5/BET-B study of LENZ was initiated in October 2020 after a review of 400 compounds. Based on the strength of the LIVE-AIR results, the NIH advanced and expanded ACTIV-5/BET-B up to 550 patients. Additionally, the NIH modified its Primary endpoint to assess the impact on patients with a baseline C-reactive Protein (CRP) of <150mg/L. This patient population is currently not served by other immunomodulatory therapies. NIH has fully enrolled the study and is now in the process of data analysis.
1. Temesgen Z, et al. Lancet Respir Med. 2021 Dec 1:S2213-2600(21)00494-X. DOI: 10.1016/S2213-2600(21)00494-X
2. Kilcoyne A et al, (2022). Journal of Medical Economics, 25:1, 160-171, DOI: 10.1080/13696998.2022.2030148
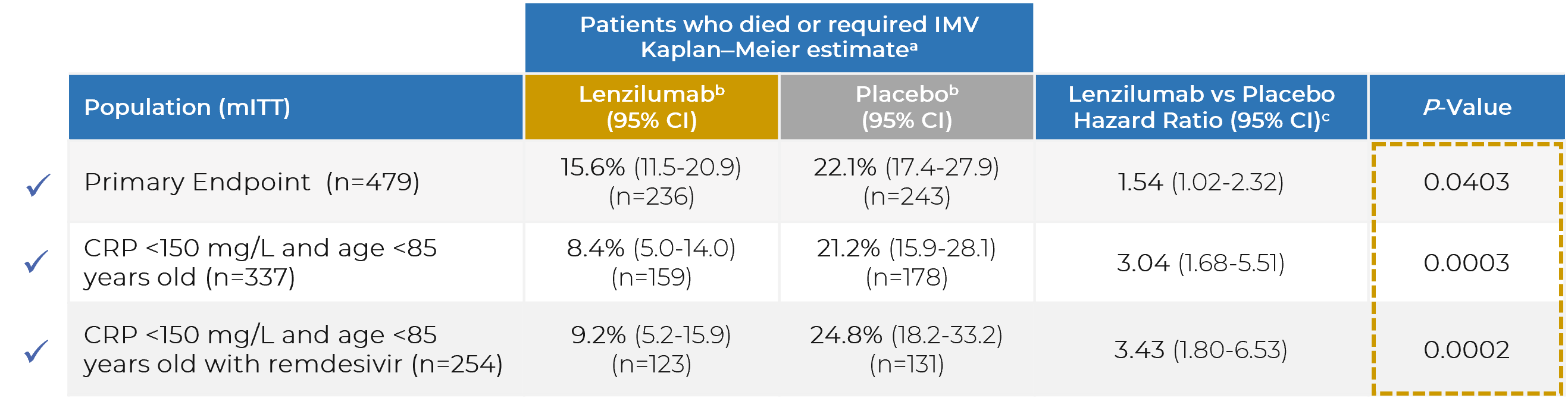
Note: CI = confidence interval; CRP = C-reactive protein; IMV = invasive mechanical ventilation; mITT = modified intent-to-treat
a. All data censored at 28 days following enrolment. All data reported for the mITT population and subgroups of the mITT population.
b. Kaplan-Meier estimates for proportion of patients.
c. Cox Proportional Hazard Model for time to event.